Research
Research Overview
Our group investigates the complex and dynamic interactions within the tumor microenvironment to understand how cancer evades immune surveillance and develops resistance to therapy. We utilize cutting-edge spatial biology tools, advanced in vitro functional assays, and diverse models to identify molecules that drive immune evasion and disease progression. Ultimately, our research aims to develop innovative therapies to combat cancer.
Current Research Projects
1. Spatial Proteogenomic Profiling of Cancer
Traditional methods for tumor profiling typically require tissue dissociation, where tumors are physically disrupted and enzymatically digested. Only after this harsh and often destructive process can we analyze the genes and proteins expressed by individual cells. As a result, most transcriptomic and proteomic profiles generated using these methods are incomplete and lack critical spatial context.
Spatial biology tools, by contrast, enable the analysis of intact tissues, providing a more comprehensive and nuanced view of the tumor microenvironment.
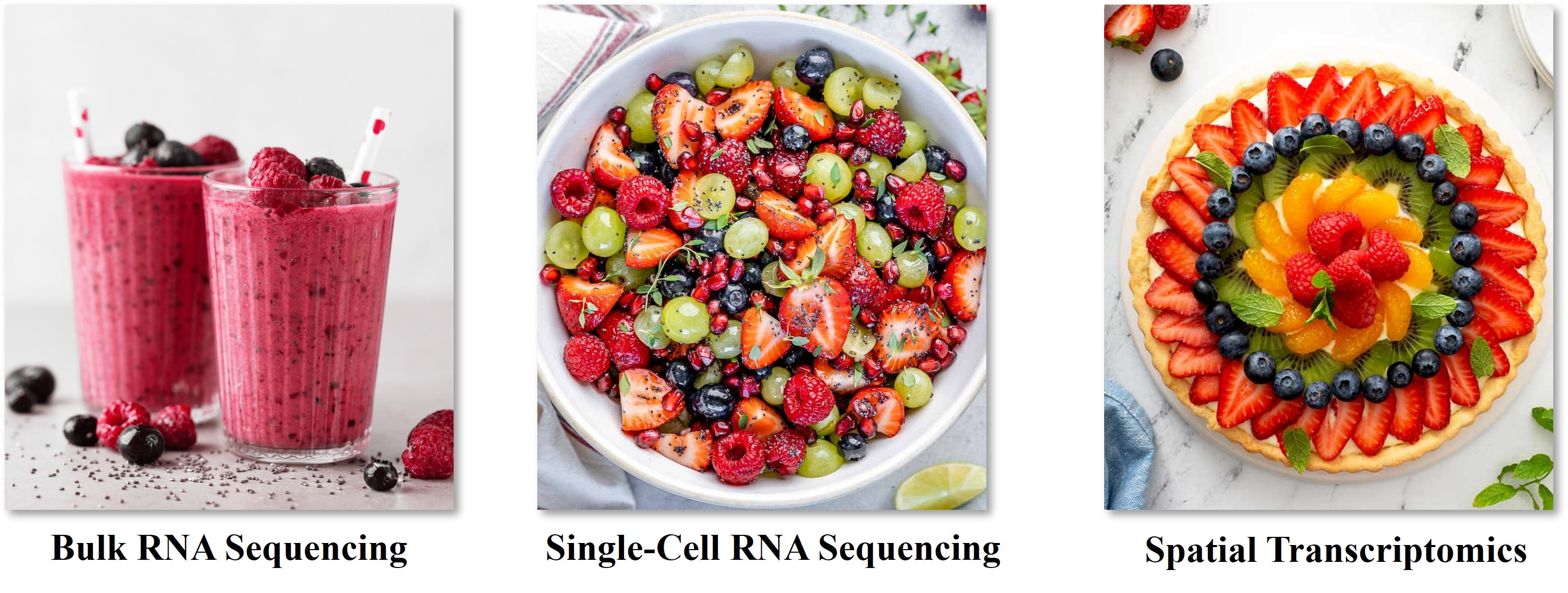
A helpful analogy—originally coined by Hongkui Zeng and Bosiljka Tasic—compares these approaches to different fruit-based dishes:
Bulk sequencing is like a fruit smoothie: all cellular components are blended together, and only the most abundant signals are detectable.
Single-cell RNA sequencing is like a fruit salad: you can identify the types and relative abundance of cells, but lose all information about their original location and interactions.
Spatial transcriptomics and proteomics (collectively known as spatial proteogenomics) are akin to a fruit tart: not only can you see which components are present, but also how they are arranged, and who their neighbors are—revealing the architectural and interactive landscape of the tumor.
This spatially-resolved approach is revolutionizing our ability to understand the complexity of tumors, their cellular interactions, and ultimately, their behavior.
2. Identification of T cell modulators in melanoma and non-small cell lung cancer
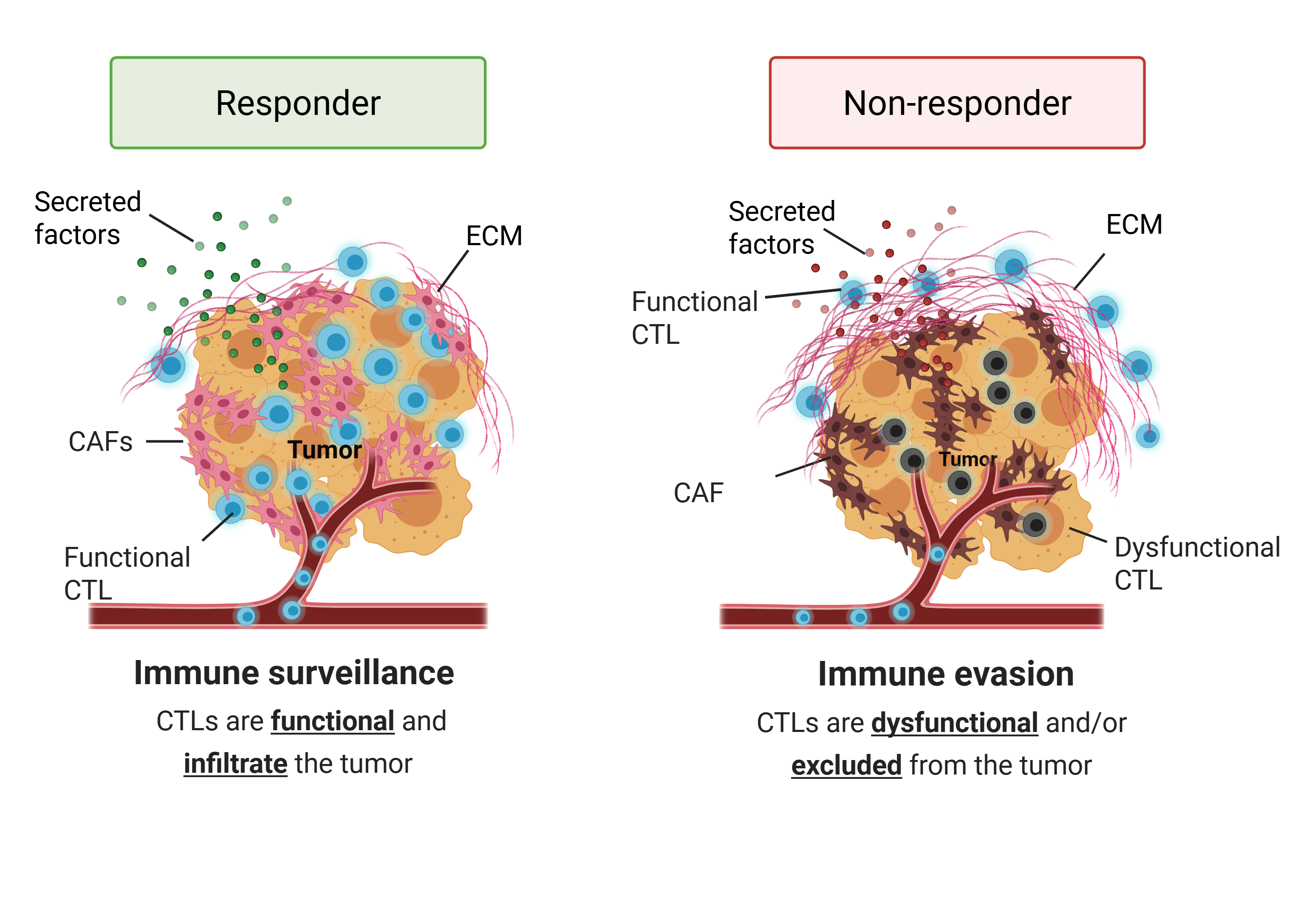
Immunotherapy is currently the most effective and durable treatment for both melanoma and non-small cell lung cancer. However, only a subset of patients benefits from this approach. The majority are non-responders—patients whose tumors are resistant to immunotherapy.
One major contributor to this resistance is the absence or scarcity of functional cytotoxic T lymphocytes (CTLs) within the tumor microenvironment. CTLs are critical immune cells responsible for directly killing cancer cells. Their absence may result from CTL exclusion (where immune cells are unable to infiltrate the tumor) and/or CTL dysfunction (where infiltrating cells are rendered inactive by inhibitory signals such as immune checkpoints).
Uncovering the mechanisms underlying CTL exclusion and dysfunction could enable us to restore immune surveillance—the body's natural ability to detect and eliminate cancer cells—and ultimately improve patient outcomes.
Recent Publications
- •Kidacki, M., Cho, C., Lopez-Giraldez, F., Huang, B., He, J., Gaule, P., Chen L., and Vesely, M., PD-1H (VISTA) expression in cutaneous squamous cell carcinoma is correlated with T cell infiltration and activation. JID. 2024 February 19. https://doi.org/10.1016/j.jid.2025.01.030
- •Kidacki, M., Cho, C., Lopez-Giraldez, F., Breidbart, R., Jaiswal, A., Damsky, W., Chen, L., Vesely, MD., Spatial transcriptomics reveals differential inflammatory pathways in discoid lupus erythematosus and lichen planus. JID. 2025 March 18. doi: 10.1016/j.jid.2025.02.148
- •Cho, C., Haddadi, NZ., Woodard, GA., Richmond, JM., Vesely, MD., Digital Spatial profiling of proteins and genes in inflammatory skin disease. JID Innovations. 2024 September; 26 (4). https://doi.org/10.1016/j.xjidi.2024.100317
- •Woodard GA, Cho C, Chen L. Increased Lymphocyte Infiltration in NSCLC Neoadjuvant Chemo-Immunotherapy Non-responders: A Biomarker of T-Cell Dysfunction and Prognosis?. Ann Surg Oncol. 2024 Jan;31(1):25-27. doi: 10.1245/s10434-023-14388-1. Epub 2023 Oct 29. PubMed PMID: 37899411.
- •Lu Z, Bae EA, Verginadis II, Zhang H, Cho C, McBrearty N, George SS, Diehl JA, Koumenis C, Bradley LM, Fuchs SY. Induction of the activating transcription factor-4 in the intratumoral CD8+ T cells sustains their viability and anti-tumor activities. Cancer Immunol Immunother. 2023 Apr;72(4):815-826. doi: 10.1007/s00262-022-03286-2. Epub 2022 Sep 5. PubMed PMID: 36063172; PubMed Central PMCID: PMC10317204.
- •McBrearty N*, Cho C*, Chen J, Zahedi F, Peck AR, Radaelli E, Assenmacher CA, Pavlak C, Devine A, Yu P, Lu Z, Zhang H, Li J, Pitarresi JR, Astsaturov I, Cukierman E, Rustgi AK, Stanger BZ, Rui H, Fuchs SY. Tumor-Suppressive and Immune-Stimulating Roles of Cholesterol 25-hydroxylase in Pancreatic Cancer Cells. Mol Cancer Res. 2023 Mar 1;21(3):228-239. doi: 10.1158/1541-7786.MCR-22-0602. PubMed PMID: 36378658; PubMed Central PMCID: PMC9992122. (*equally contributed; co-first authors)
- •Cho C, Mukherjee R, Peck AR, Sun Y, McBrearty N, Katlinski KV, Gui J, Govindaraju PK, Puré E, Rui H, Fuchs SY. Cancer-associated fibroblasts downregulate type I interferon receptor to stimulate intratumoral stromagenesis. Oncogene. 2020 Sep;39(38):6129-6137. doi: 10.1038/s41388-020-01424-7. Epub 2020 Aug 17. PubMed PMID: 32807917; PubMed Central PMCID: PMC7502515.
- •Cho C, Horzempa C, Longo CM, Peters DM, Jones DM, McKeown-Longo PJ. Fibronectin in the Tumor Microenvironment Activates a TLR4-dependent Inflammatory Response in Lung Cancer Cells. J Cancer. 2020;11(11):3099-3105. doi: 10.7150/jca.39771. eCollection 2020. PubMed PMID: 32231714; PubMed Central PMCID: PMC7097952.
- •Cho C, Horzempa C, Jones D, McKeown-Longo PJ. The fibronectin III-1 domain activates a PI3-Kinase/Akt signaling pathway leading to αvβ5 integrin activation and TRAIL resistance in human lung cancer cells. BMC Cancer. 2016 Aug 2;16:574. doi: 10.1186/s12885-016-2621-6. PubMed PMID: 27484721; PubMed Central PMCID: PMC4970220.
Funding & Support
- •Robert E. Leet and Clara Guthrie Patterson Trust Mentored Research Award (2023-2025)
- •Department of Defense Health Program Congressionally Directed Medical Research Programs Melanoma Research Program Idea Award (2025-2027)

